Hi folks,
In the last blog "Determining conditions for Primary drying of Lyophilization- Part I" we have seen the utilization of Differential scanning calorimetry (DSC) to determine the glass transition temperature of formulation, that helps us to set shelf temperature during primary drying of lyophilization of pharmaceuticals.
Now lets see other useful information that can be obtained from DSC.
If the sample run through DSC, it makes sense that every possible information shall be obtained. Hence, lets have a glance on those information.
Crystallization:
A Sample after glass transition, substances will have a lot of mobility and never stay in one position for very long time. But when they reached a specific temperature, it will give off enough energy to move into very ordered arrangements, which are called crystalline substances and they release heat. So it doesn't have to put out much heat to keep the temperature of the sample pan rising. This drop in the heat flow as a big peak in the plot of heat flow vs. temperature.
The temperature at the highest point in the peak is usually considered to be the crystallization temperature, or Tc. Also, the area of the peak can be measured, which tells us the latent energy of crystallization of the substance. But most importantly, this peak tells us that the substance can in fact crystallize. If 100% amorphous polymer is analysed, like polystyrene, this peak cannot be obtained, because such materials don't crystallize also, because the polymer gives off heat when it crystallizes, called as crystallization is an exothermic transition.
Also, liquid formulation during freezing step once crystallization occurs that may result in little sharp peak from base line of a DSC as shown in above figure.
Now lets see, what happens when the substance gets heated beyond its crystallization temperature. The resulting thermal transition is called Melting.
Melting:
When substance's melting temperature is reached, polymer crystals begin to fall apart, that is they melt. It comes out of their ordered arrangements, and begin to move around freely that can be spotted on a DSC
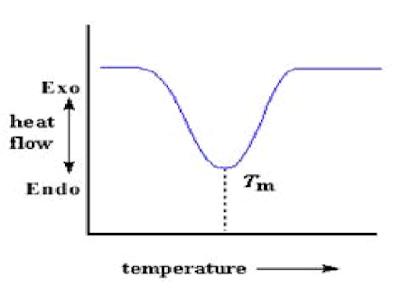
plot The heat which polymer give off when crystallized is absorbed when reached at Tm. That is a latent heat of melting like latent heat of crystallization. When the polymer crystals melt, they must absorb heat in order to do so. Melting is a first order transition. This means that at the melting temperature, the polymer's temperature won't rise until all the crystals have melted. The heater under the sample pan has to put a lot of heat into the polymer in order to both melt the crystals and keep the temperature rising at the same rate as that of the reference pan. This extra heat flow during melting shows up as a big dip on DSC plot, like this:
In a DSC curve, all the three phase transitions seen above Glass transition, Crystallization, melting are denoted in a single curve as shown below.
Before concluding the topic of DSC, for a better understanding on variations in DSC curves due to crystallization and melting process are provided below.
Conclusion:
Even though DSC is useful for Tg determination, it should not be the one and only evaluation to determine the primary drying temperature. As any method has its own uses and limitatiions, DSC has below limitations.
That's all for today folks. Hope the content was useful.
Take care......
Regards,
Teja Ponduri
In the last blog "Determining conditions for Primary drying of Lyophilization- Part I" we have seen the utilization of Differential scanning calorimetry (DSC) to determine the glass transition temperature of formulation, that helps us to set shelf temperature during primary drying of lyophilization of pharmaceuticals.
Now lets see other useful information that can be obtained from DSC.
If the sample run through DSC, it makes sense that every possible information shall be obtained. Hence, lets have a glance on those information.
Crystallization:
A Sample after glass transition, substances will have a lot of mobility and never stay in one position for very long time. But when they reached a specific temperature, it will give off enough energy to move into very ordered arrangements, which are called crystalline substances and they release heat. So it doesn't have to put out much heat to keep the temperature of the sample pan rising. This drop in the heat flow as a big peak in the plot of heat flow vs. temperature.
The temperature at the highest point in the peak is usually considered to be the crystallization temperature, or Tc. Also, the area of the peak can be measured, which tells us the latent energy of crystallization of the substance. But most importantly, this peak tells us that the substance can in fact crystallize. If 100% amorphous polymer is analysed, like polystyrene, this peak cannot be obtained, because such materials don't crystallize also, because the polymer gives off heat when it crystallizes, called as crystallization is an exothermic transition.
Also, liquid formulation during freezing step once crystallization occurs that may result in little sharp peak from base line of a DSC as shown in above figure.
Now lets see, what happens when the substance gets heated beyond its crystallization temperature. The resulting thermal transition is called Melting.
Melting:
When substance's melting temperature is reached, polymer crystals begin to fall apart, that is they melt. It comes out of their ordered arrangements, and begin to move around freely that can be spotted on a DSC
plot The heat which polymer give off when crystallized is absorbed when reached at Tm. That is a latent heat of melting like latent heat of crystallization. When the polymer crystals melt, they must absorb heat in order to do so. Melting is a first order transition. This means that at the melting temperature, the polymer's temperature won't rise until all the crystals have melted. The heater under the sample pan has to put a lot of heat into the polymer in order to both melt the crystals and keep the temperature rising at the same rate as that of the reference pan. This extra heat flow during melting shows up as a big dip on DSC plot, like this:
In a DSC curve, all the three phase transitions seen above Glass transition, Crystallization, melting are denoted in a single curve as shown below.
Even though DSC is useful for Tg determination, it should not be the one and only evaluation to determine the primary drying temperature. As any method has its own uses and limitatiions, DSC has below limitations.
- can not really control the rate of experiment (can only be checked but cant controlled)
- Dependent on too many parameters (Thorough understanding of analysis is required for analyst)
- Very sensitive to any changes
- Result depends a lot from the operator (Well trained operator required)
That's all for today folks. Hope the content was useful.
Take care......
Regards,
Teja Ponduri




No comments:
Post a Comment